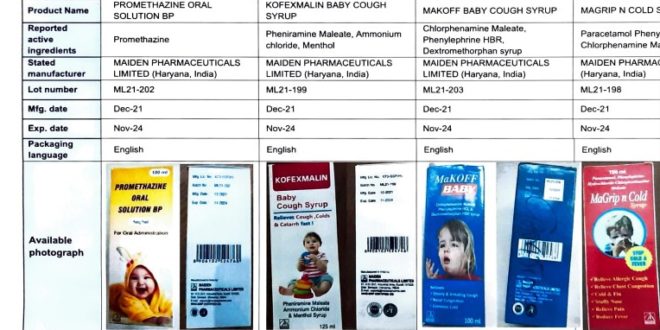
The Food and Drugs Authority (FDA) has announced that the World Health Organization (WHO) has identified four substandard medicinal products in The Gambia.
The four products are; Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
According to a statement from the FDA, laboratory analysis of the products confirms that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
The FDA explained that diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal.

The contaminated paediatric medicines identified by the WHO
Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.
The manufacturer of these products has been identified as Maiden Pharmaceuticals Limited based in Haryana, India.
The statement added that the manufacturers, Maiden Pharmaceuticals Limited as of October 5 has still not provided safety and quality guarantee of these products to the WHO.
“The FDA would like to inform you that these products have not been registered by the Authority and are not expected on the Ghanaian market, however, they may have been distributed illegally,” the Authority said in a statement on October 6, 2022.
The Authority has also advised all healthcare professionals to report suspected falsified medicinal products to the FDA using the link http://adr.fdaghana.gov.gh or call mobile number on 024431 0297.
Meanwhile, the FDA has strengthened its post market surveillance activities at the borders and across the country with the view to identify and withdraw any unregistered products on the Ghanaian market.
Thanks for reading from TodayGhanaMedia.com as a news publishing website from Ghana.
Source: TodayGhanaMedia.
Are You Suffering From Weak Erection, Low Libido, Premature Ejaculation Or Infections? – Get M-Plus No

Maccun Plus (MPlus) is for men and women as a natural aphrodisiac with no side effects
Just contact the number below for M PLUS HONEY.
 TodayGhanamedia.com Serving With Integrity & Professionalism
TodayGhanamedia.com Serving With Integrity & Professionalism